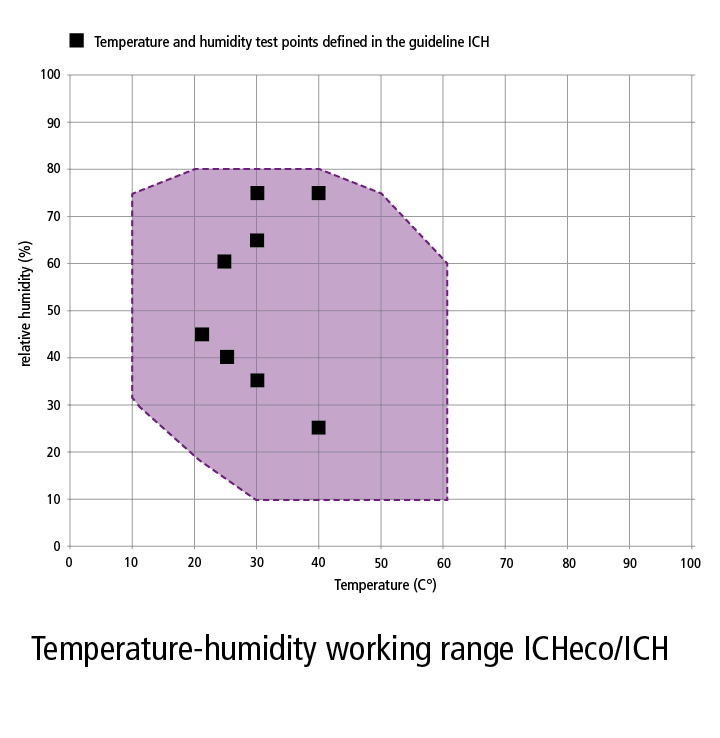
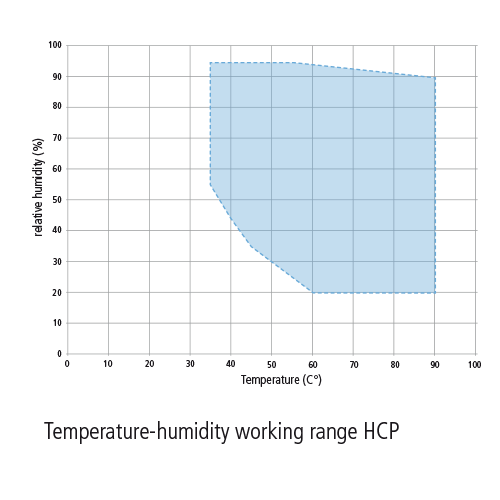
The csa sterilization standards committee has recommended that the range for acceptable relative humidity for sterile storage should be 30 70 unless contraindicated by device manufacturer.
Sterile storage temperature and humidity.
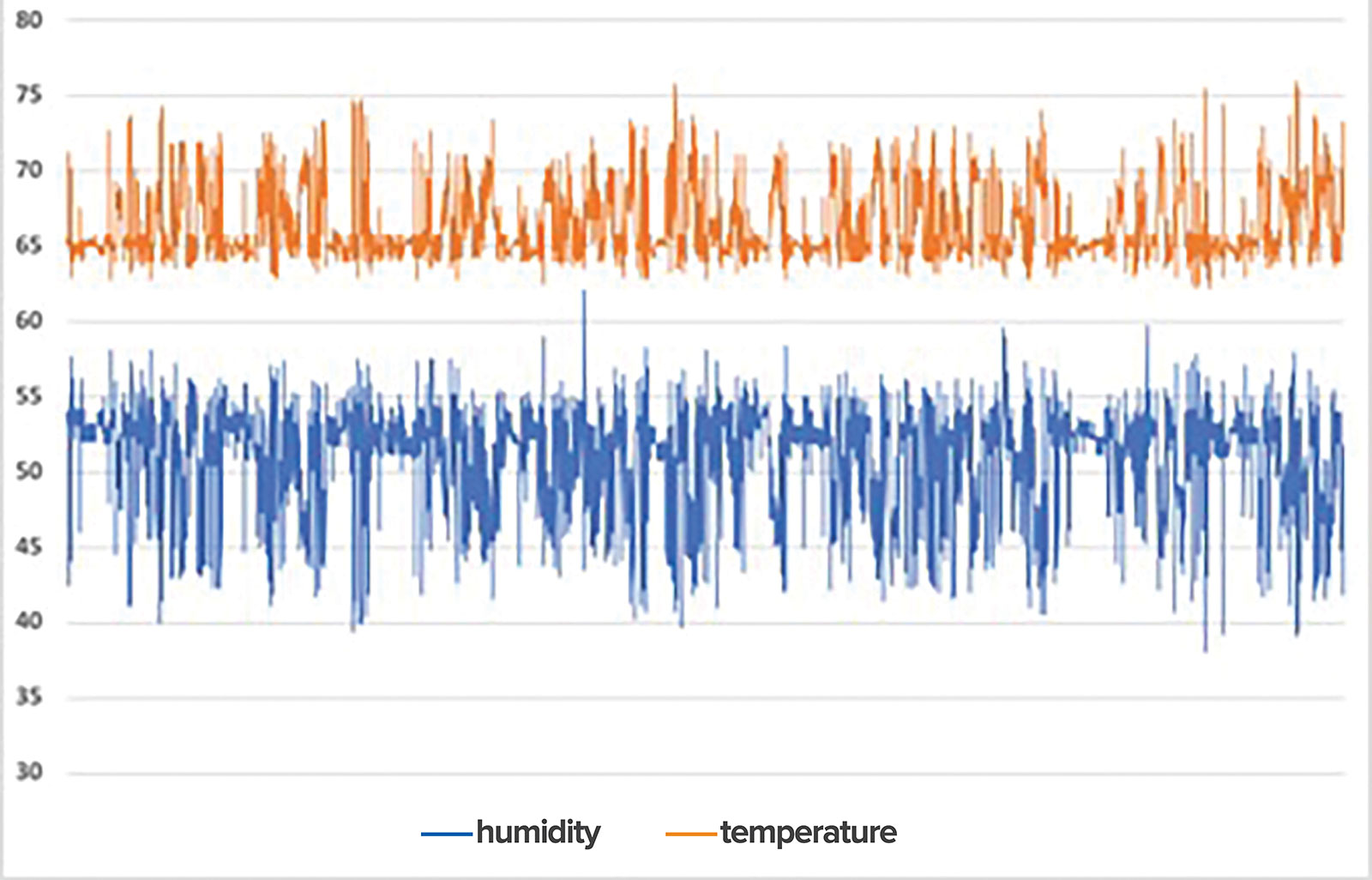
Temperatures between 18 22 c and relative humidity rh between 35 68 1.
819 the floors and walls should be constructed of materials capable of withstanding chemical agents used for cleaning or disinfecting.
Sterile medical devices smd irrespective of whether these.
By its very nature the risk assessment will inform all team members of the appropriate response to the parameter variance and ensure patient safety.
Hot temperatures can ruin the materials or break the seals in original packaging.
The sterile storage area should be a limited access area with a controlled temperature may be as high as 75 f and relative humidity 30 60 in all works areas except sterile storage where the relative humidity should not exceed 70.
Items frequently found in sterile processing requiring a specific temperature and or humidity range include wrapping materials biological indicators chemical indicators and bowie dick tests.
Conditions for smd storage require.
Sterile storage temperature and humidity parameters.
Current guidelines provide ranges for acceptable humidity levels in healthcare sterile storage areas of 30 60 relative humidity.
Centralized sterile supply storage in procedural areas where large volumes of sterile supplies are kept require a program for management of proper temperature and relative humidity rh levels in accordance with the facilities guidelines institute fgi guidelines for design and construction of hospitals and outpatient facilities.
Are produced by a sterile services department ssd or a.