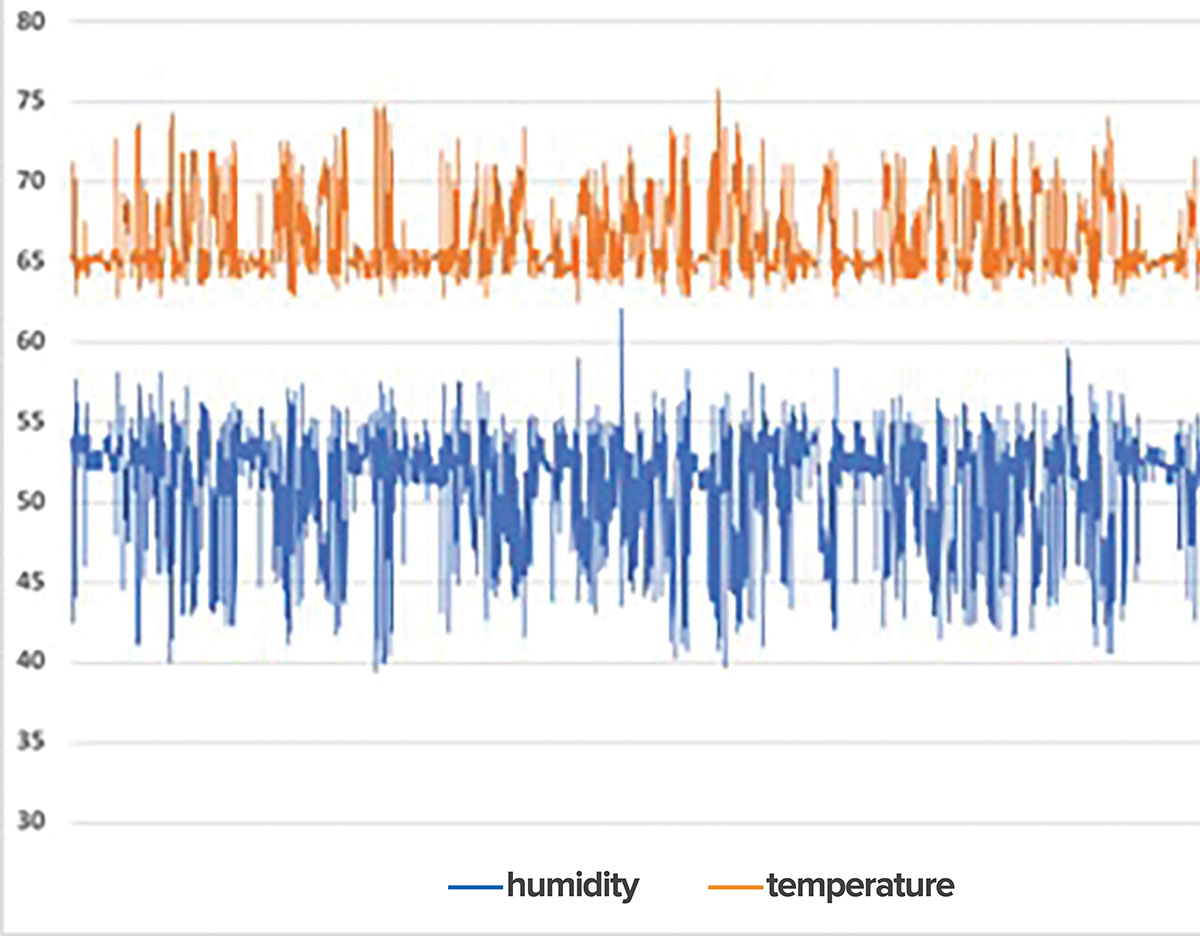
Current guidelines provide ranges for acceptable humidity levels in healthcare sterile storage areas of 30 60 relative humidity.
Sterile instrument storage temperature and humidity.
819 the floors and walls should be constructed of materials capable of withstanding chemical agents used for.
Excessive humidity can also cause compromise of sterility.
Moisture buildup degrades fibrous material and can create a breeding ground for even.
Examples of products that are sensitive to humidity include biological and chemical indicators and ekg electrodes.
High relative humidity in sterile storage areas prepared august 1 2007 by an ad hoc committee of sterilization experts attending a canadian standards association meeting introduction.
The sterile storage area should ideally be physically separated enclosed located close to the sterilization area and dust free with at least four air exchanges per hour.
Centralized sterile supply storage in procedural areas where large volumes of sterile supplies are kept require a program for management of proper temperature and relative humidity rh levels in accordance with the facilities guidelines institute fgi guidelines for design and construction of hospitals and outpatient facilities.
The humidity level measured with a hygrometer should range from 35 170 and temperature should not exceed 75.
The sterile storage area should be a limited access area with a controlled temperature may be as high as 75 f and relative humidity 30 60 in all works areas except sterile storage where the relative humidity should not exceed 70.
Therefore shelf life and product integrity can be even more greatly affected if the ifu calls for 30 60 rh but the hdo lowers the rh to 20.
Soil can also compromise sterility.
Please see ansi aami st79 2017 comprehensive guide to steam sterilization and sterility assurance in health care facilities for more information sections 3 2 1 1 3 3 5 5 and 3 3 6 1 1.
Sterile storage temperature and humidity parameters.
Hot temperatures can ruin the materials or break the seals in original packaging.
Excessive humidity levels in the sterile storage area or handling packages too soon after sterilization can also affect sterility.
Terry mcauley msc medical device decontamination current grad dip education and training steam consulting melbourne victoria po box 100 endeavour hills vic.
Humidity k design temperature l f c central medical and surgical supply sterile processing departmentz soiled or ddecontamination room negative 2 6 yes no nr 72 78 22 26 60 73 16 23 clean workroom positive 2 4 nr no max 60 72 78 22 26 68 73 20 23 sterile storage positive 2 4 nr nr max 60 72 78 22 26 max 75 24 notes.
Affects the shelf life and product integrity of sterile supplies.
Maintaining the right temperature and humidity conditions is essential for protecting goods from contaminants and degradation.